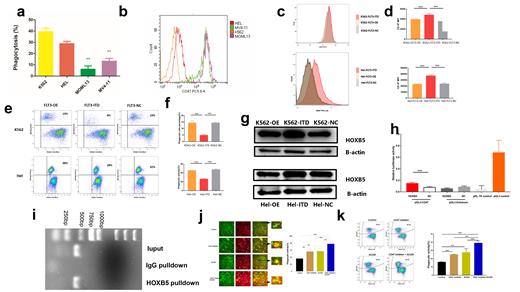
The FLT3-ITD mutation is frequently observed in acute myeloid leukemia (AML) and confer a poor prognosis. FLT3 inhibitors such as Quizartinib could prolong survival. However, very few relapsed patients are cured. Little attention has been paid to the characteristics of the immune microenvironment in FLT3-ITD AML. Within the bone marrow, Flt3/ITD animals demonstrated significantly increased dendritic cells and T cells levels compared to Flt3/WT mice, which suggested that FLT3-ITD can modulate the immune microenvironment in AML. Here, we found that FLT3-ITD cell lines (MV-4-11 and MOLM-13) induced less LDH release compared to FLT3-WT cell lines (HEL and K562) ( Figure a), indicating less macrophage killing. CD47 gene is an integrin related protein, which acts as an “anti phagocytosis” signal, and it interacts with signal-regulatory protein alpha (SIRPalpha) on macrophages, so that tumor cells expressing CD47 can escape being destroyed by macrophages. Further Flow Cytometry results showed that the CD47 Mean fluorescent intensity (MFI) of the FLT3-ITD group was significantly higher than those of the control group ( Figure b). Therefore, we can ask a hypothesis that FLT3-ITD in tumor cells may leads to decreased phagocytic capacity of macrophages and immune tolerance. To test our hypothesis, the FLT3 wildtype (FLT3-OE, FLT3 over expression) or ITD mutant (FLT3-ITD) stable overexpression HEL/K562 cells were constructed by means of lentiviral transfections. In the FLT3-ITD group, the MFI levels of CD47 was higher than FLT3-OE group and FLT3 NC (FLT3 Normal control) group ( Figure c and d). Green-fluorescent (CFSE-stained) Hel and K562 were then added to cultures of macrophages induced by Peripheral Blood Mononuclear Cell stained with the red dye PKH26 (ratio tumor cell:macrophages, 5:1). Flow Cytometry results showed FLT3-ITD decreased the Phagocytic Capacity of Macrophages ( Figure e and f). The TRANSFAC database was used to predict the transcription factor binding sites of the CD47 promoter. We found that HOXB5 may regulate the expression of CD47 at the transcriptional level. Compared with the FLT3-NC and FLT3-OE group, the relative expression level of the CD47 gene in the FLT3-ITD group was significantly upregulated ( Figure g, p < 0.05). To ascertain the mechanism that HOXB5 regulated CD47, we analysed the location CD47 gene on the chromosome, as well as the sequence of promoter and first non-encoding exon of HOXB5 (−2000 ~ 100 bp) by Jaspar online tool (http://jaspar.genereg.net/). Mechanistically, Dual luciferase reporter gene assay and ChIP experiments suggest HOXB5 directly activate CD47( Figure h and i). Compared to the monotherapy group, Combination therapy with CD47 inhibitor and Quizartinib (AC220) significantly promoted the phagocytic ability of M1-like macrophages against MOLM13 cells ( Figure j and k). The mouse model of AML was constructed by injection of 5x106 FLT3-ITD cells via the tail vein. Mouse models further demonstrate that combination therapy with CD47 inhibitor and Quizartinib significantly reduces the tumor burden and improves survival rate. We first revealed that FLT3-ITD can up-regulates CD47 to promote immune escape of macrophages. These evidences indicates that combination therapy with CD47 inhibitor and FLT3-ITD inhibitor can be a promising therapeutic method for impressive treatment of FLT3-ITD AML patients.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal